Summary of the study
To advance research and improve the management of resistant hypertension: participate in the clinical trial
To advance research and improve the management of resistant hypertension: participate in the clinical trial

" Medico-economic evaluation of unilateral carotid sinus stimulation in the treatment of resistant hypertension: a multicentre, randomised, open-label, controlled trial with blood pressure efficacy and safety assessment (IDRCB: 2014-A00632-45)"
The CHRU of NANCY and several Centres of Excellence in Arterial Hypertension of the French Society of Arterial Hypertension invite you to participate in the ESTIM-rHTN clinical trial promoted by the CHRU of Nancy and approved by the health authorities.
This study, which has the support of the Ministry of Health, aims to evaluate the efficacy and safety of an implantable medical device Barostim neo™in hypertension compared to usual management.
This study will also make it possible to evaluate the place and interest that the Barostim neo™implantable medical device can take in relation to exclusive drug treatment with regard to the management of this disease already existing in France.
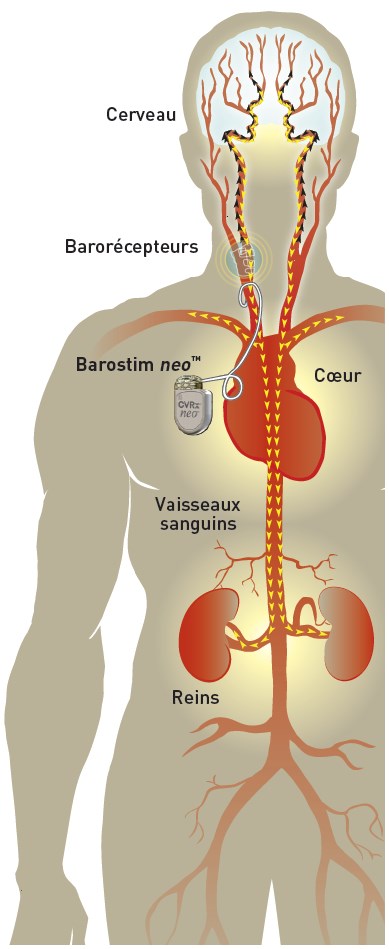 The system Barostim neo™ is a CE marked implantable device designed to stimulate the body's natural cardiovascular regulators called "baroreceptors" (blood pressure receptors) thereby lowering blood pressure.
The system Barostim neo™ is a CE marked implantable device designed to stimulate the body's natural cardiovascular regulators called "baroreceptors" (blood pressure receptors) thereby lowering blood pressure.
It consists of placing a small pellet (electrode) connected to a battery (the barostimulator) under the skin in an artery in the neck; this is called an implantable device. It is therefore a surgical treatment and is not a drug.

The pacemaker resembles the cardiac pacemakers that are commonly used to treat certain heart diseases.
The surgical procedure is short, similar to the placement of a heart pacemaker, and is reversible, meaning that it can be removed or deactivated at any time for any reason.
This study is being conducted in two groups of patients with poorly controlled hypertension despite taking antihypertensive medication: a group treated with usual antihypertensive treatments according to current recommendations and a group treated with the Barostim neo™ medical device in addition to usual antihypertensive treatments. The group allocation is random (i.e. the study group to which you will belong will not depend on you or the study doctor, but will be pre-determined by computerized randomization). You will have specialised follow-up at a study centre (maximum 7 visits) for 12 months.)
Cardiology and hypertension unit
Prof Philippe GOSSE
Contact: Ms Julie GAUDISSARD
Cardiology and Cardiovascular Rehabilitation Unit
Dr Thierry DENOLLE
Mail : estim-dinard@cic-p-nancy.fr
Department of Vascular Medicine and High Blood Pressure
Dr Pascal DELSART
By mail to the attention of Dr DELSART :
Department of Vascular Medicine and High Blood Pressure
Heart-Lung Institute, CHRU Lille
Bd Pr Leclercq, 59037 Lille Cedex
or by mail: estim-lille@cic-p-nancy.fr
Department of Cardiology, Rhythmology and Hypertension
Dr Bernard VAÏSSE
Centre for Multilingual Clinical Investigation
Prof Patrick ROSSIGNOL
Tel: 03 83 15 73 05
Mail : estim-nancy@cic-p-nancy.fr
Cardiovascular Prevention Unit
Prof Xavier GIRERD
Consultation by appointment on 01 42 17 78 32 or 01 42 17 57 74
Mail : Sylvie.maison@aphp.fr
Department of hypertension, renal and cardiovascular diseases
Prof Michel AZIZI
Website : www.centre-hypertension.org
Diagnostic and Therapeutic Centre
Prof Jacques BLACHER
Ms Veronique LABRO
Tel: 01 42 34 80 25
Clinical Investigation Centre 1402
Prof Samy HADJADJ
Tel: 05 49 44 46 89
Department of Internal Medicine and Hypertension
Prof Béatrice DULY - BOUHANICK
Mail : amar-j.sec@chu-toulouse.fr
Hypertension unit, risk factors and heart failure
Prof Atul PATHAK
Mail : apathak@clinique-pasteur.com
Department of Nephrology-HTA, Dialysis, Transplantation
Prof Jean Michel HALIMI
Mail : estim-tours@cic-p-nancy.fr
Clinical research (involving the human person) is conducted in strict compliance with the ethics and legislation in force.
This research is funded by the Ministry of Health (Medico-economic Research Programme 2013 (PRME-13-0260))